Your 510(k) Strategy,
Built on Evidence.
Stop guessing which predicates to cite. Veridocx analyzes 180,000+ cleared devices to surface the strongest matches—with complete FDA citations and audit-ready documentation.
Supported by leading programs












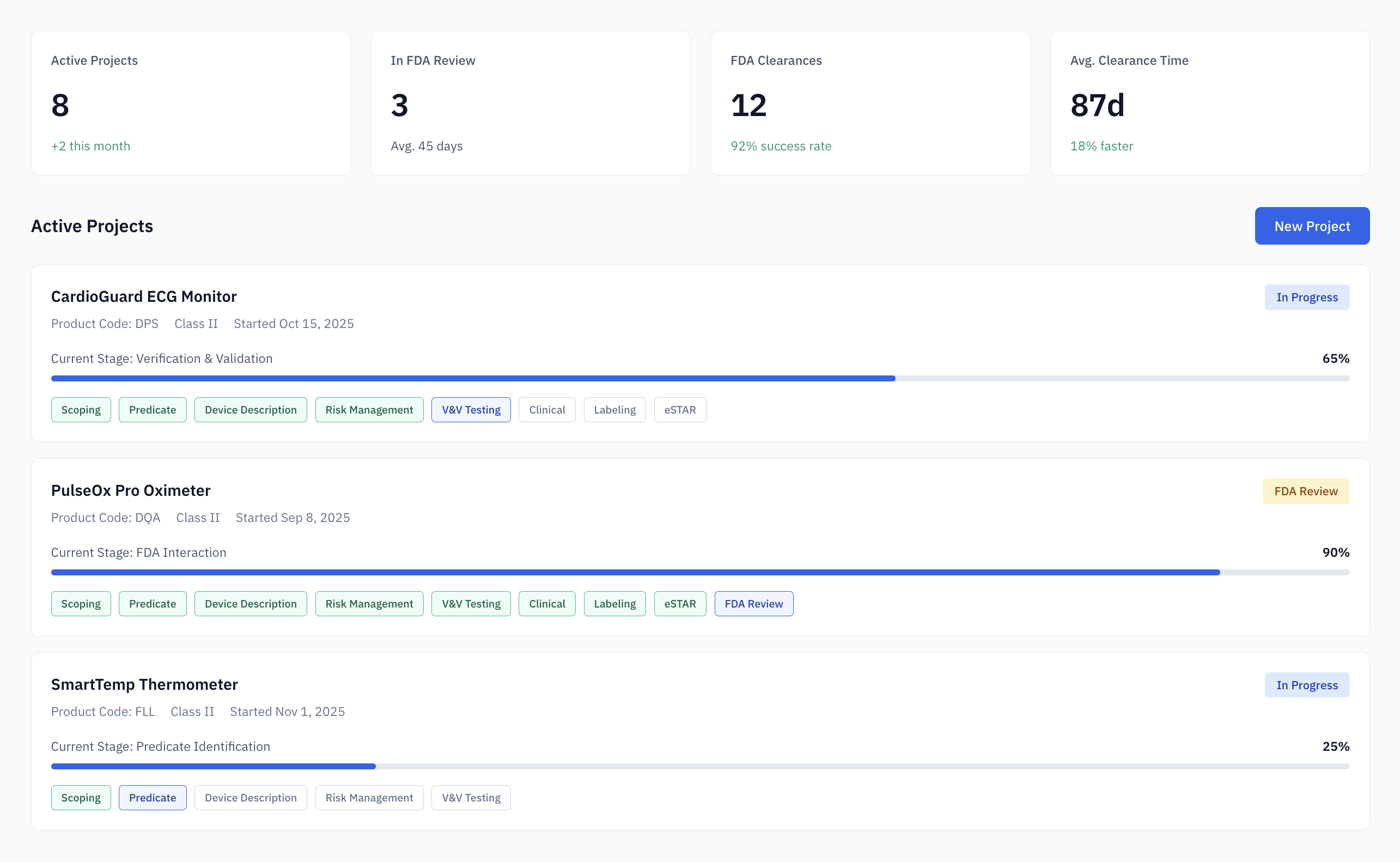
The 510(k) Problem: predictable work, high stakes.
The average 510(k) submission takes 6-12 months and costs $31,000+ in regulatory consulting fees.
Most of that time isn't spent on strategy. It's spent on systematic research—searching 180,000+ cleared devices, comparing specifications, and mapping standards.
Work that's predictable. Tedious. And ripe for automation.
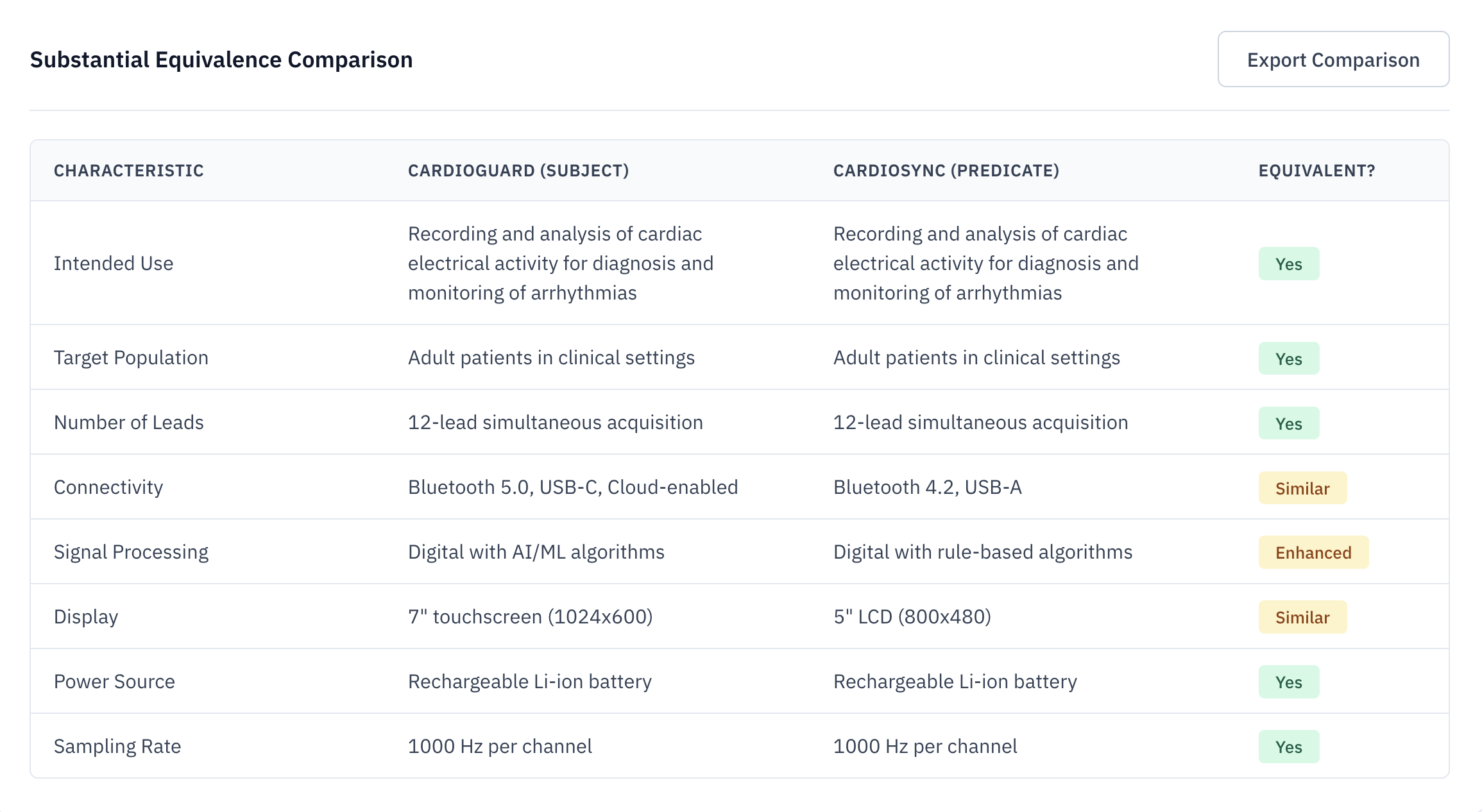
Automated SE Matrix Generation
The Substantial Equivalence (SE) matrix is the heart of your 510(k). Veridocx automates the comparison across indications, technological characteristics, and performance specifications—grounded in complete FDA citations.
Instant Comparison
Compare indications and tech specs in seconds, not days.
FDA Citations
Every data point is linked directly to the source FDA summary.
eSTAR Compatible
Export tables directly into your FDA submission templates.
Built for Regulatory Rigor
Regulatory professionals need evidence, not black-box AI. Veridocx is engineered to meet the same standards of rigor your team applies to every FDA submission.
Learn about our methodologyAlready have an eSTAR builder?
Great. Use Veridocx first.
| What You Need | Submission Tools | Veridocx |
|---|---|---|
| Find optimal predicates | Manual search | AI-ranked |
| Generate SE comparison | Write yourself | Automated |
| Identify testing gaps | Not offered | With standards |
| Fill eSTAR form | Core feature | Coming soon |
Simple Pricing
Get started free during early access. Professional plans available for teams.
Early Access
Full platform access
No credit card required
- Complete 510(k) workflow (all 5 steps)
- Access to 180,000+ 510(k) database
- Gap analysis with standards mapping
- Export in PDF, DOCX, CSV
Professional
For RA teams and consultants
Custom pricing for your team
- Everything in Early Access
- Unlimited projects
- Priority support
- Team workspaces with RBAC
- API access
Need custom deployment or compliance requirements?
Contact us for enterprise options
Ready to accelerate your 510(k) workflow?
Join regulatory teams already using Veridocx to streamline predicate research and gap analysis.
Stay Informed
Subscribe to get product updates and regulatory insights delivered to your inbox.