How Veridocx Works
From device classification to submission-ready documentation—see the complete 510(k) preparation workflow.
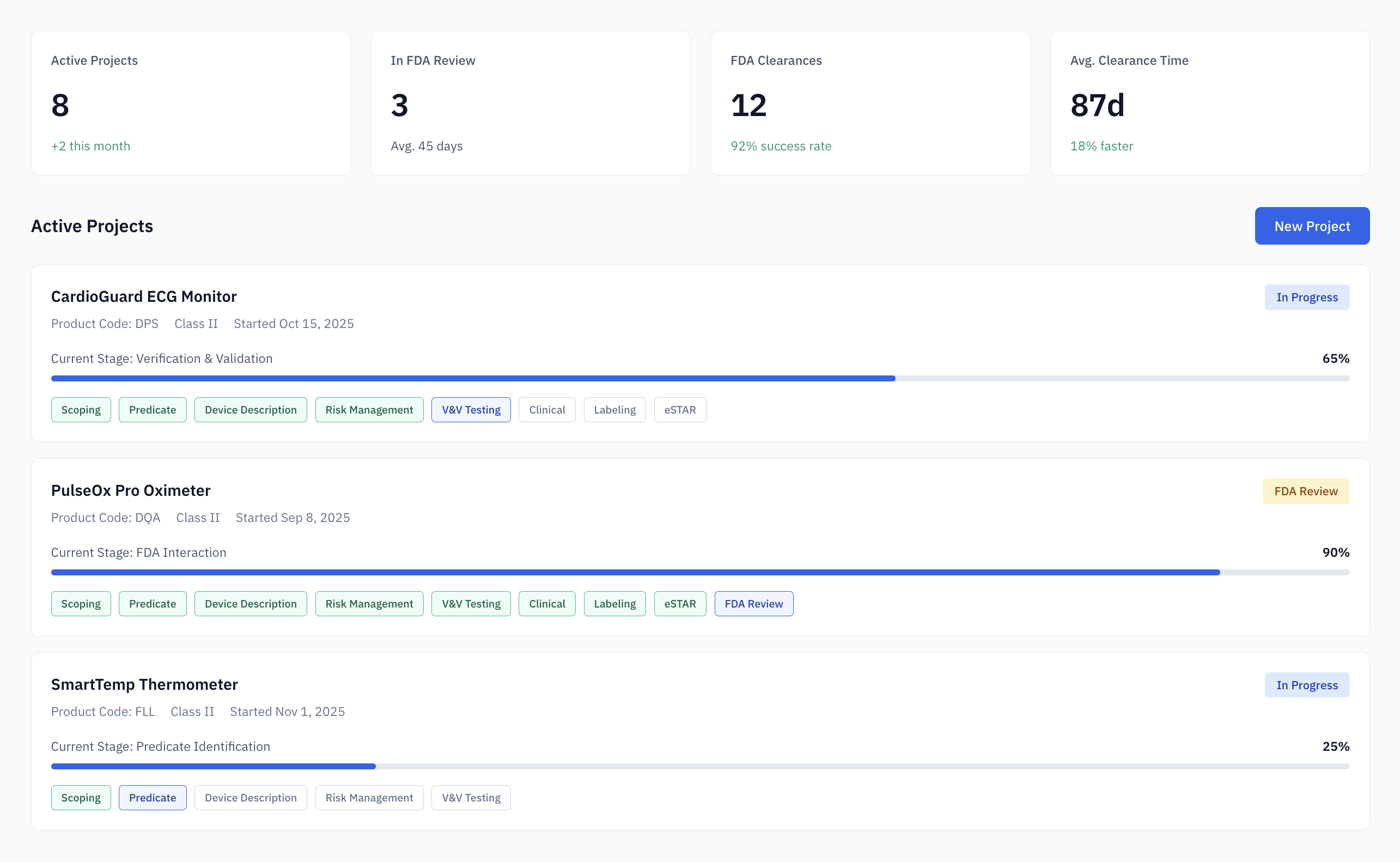
Project Dashboard
Manage multiple 510(k) projects with centralized tracking of classifications, predicate analyses, SE comparisons, and submission milestones.
- Centralized tracking
- Milestone management
- Team collaboration
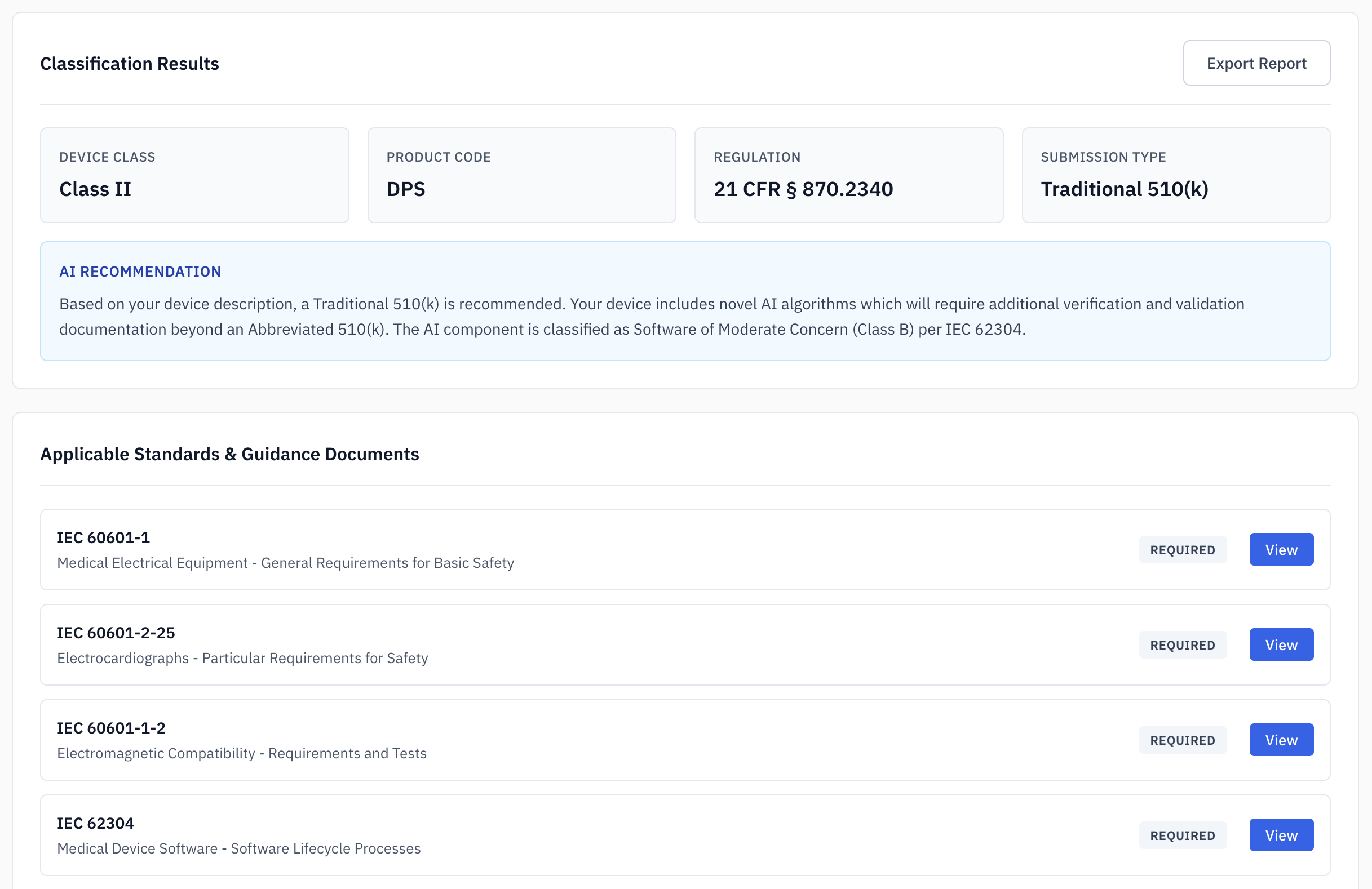
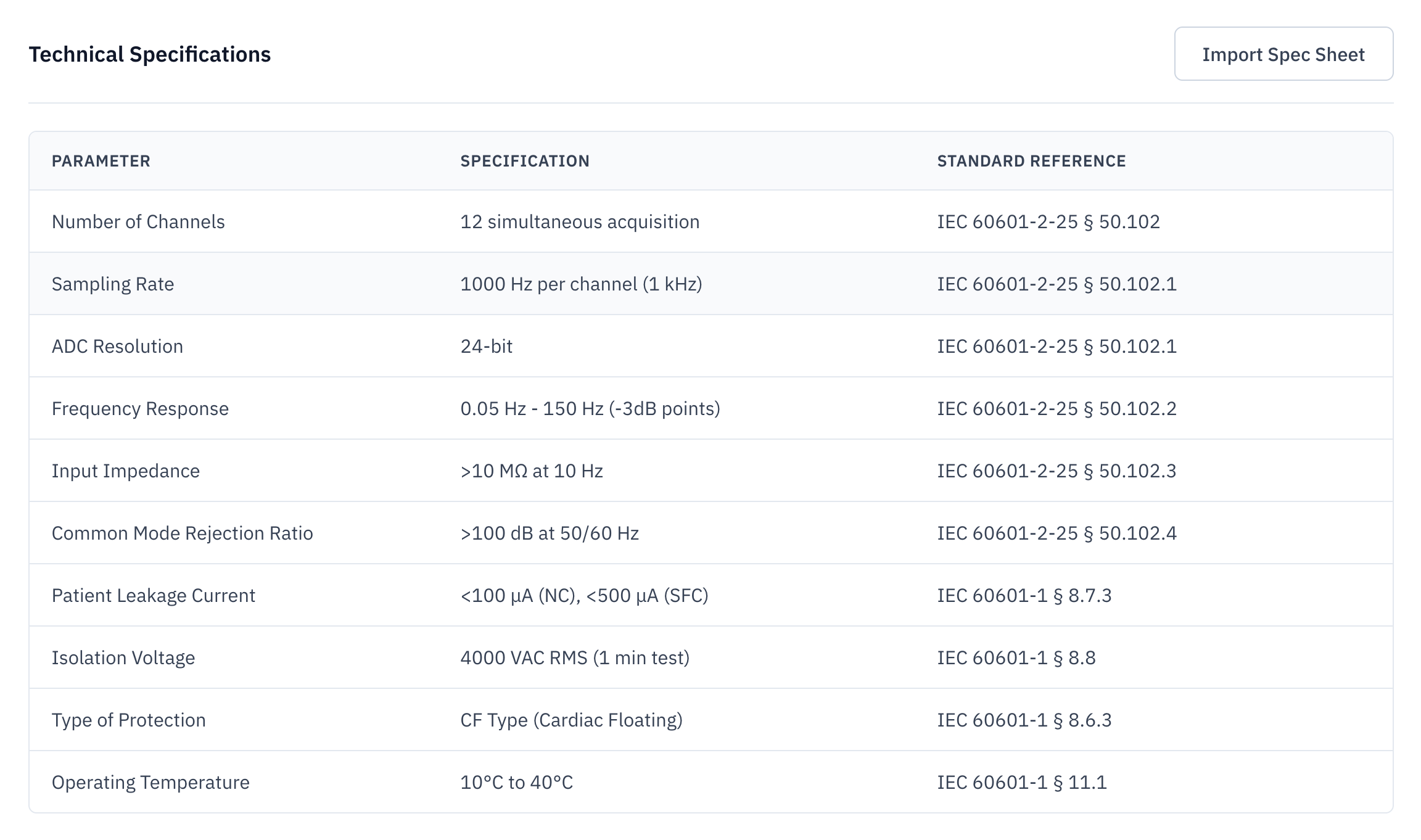
Device Classification
Determine device class and identify optimal FDA product code through systematic analysis of 21 CFR 862-892. Get AI-powered recommendations with pathway analysis, special controls, and complete citations.
- 21 CFR Analysis
- Pathway determination
- Regulatory citations
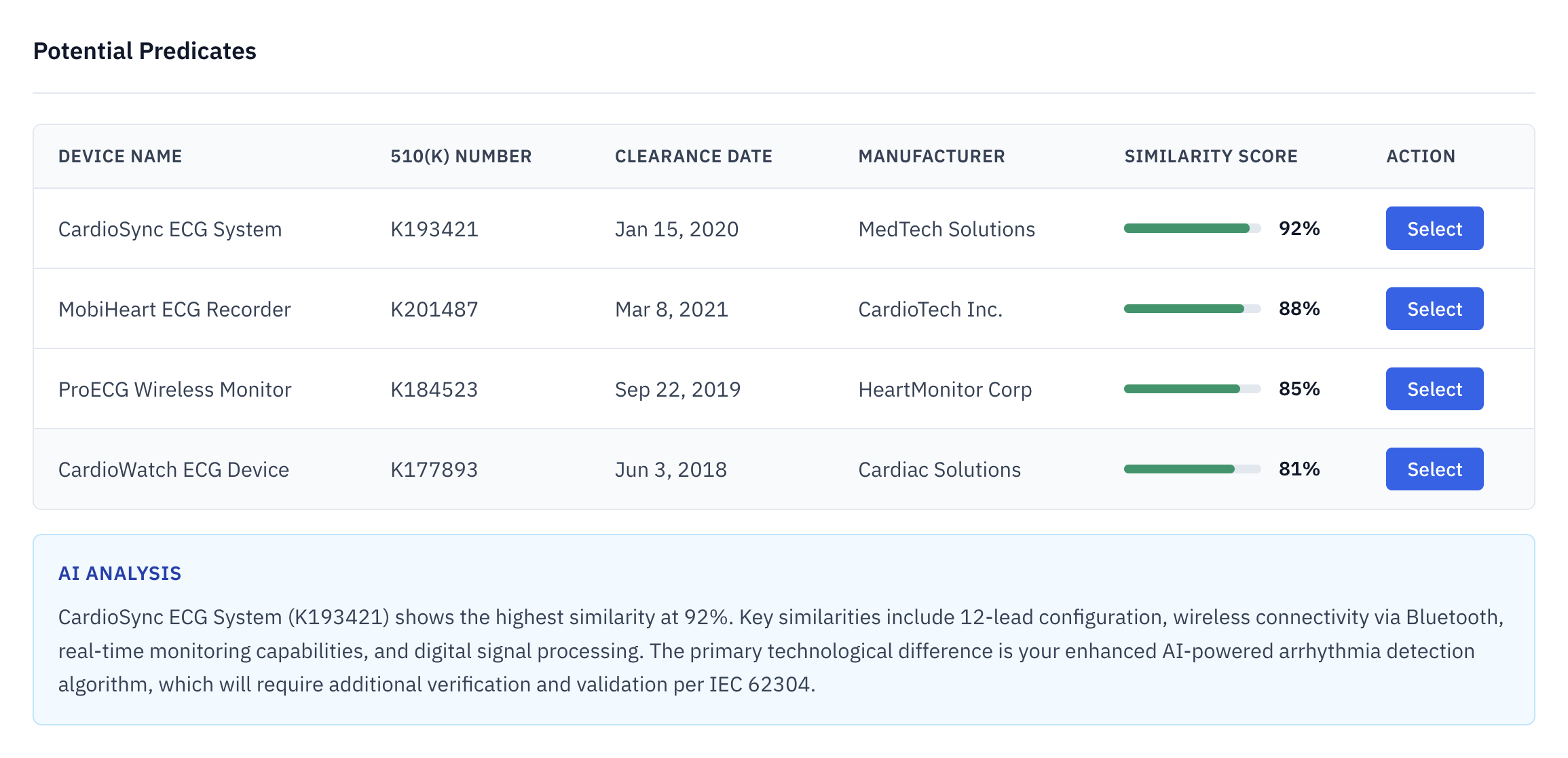
Predicate Identification
Search 180,000+ cleared 510(k) submissions to identify optimal predicates with similarity scores across indications, technology, and regulatory pathways.
- 180k+ submissions
- Similarity scoring
- Predicate chain mapping
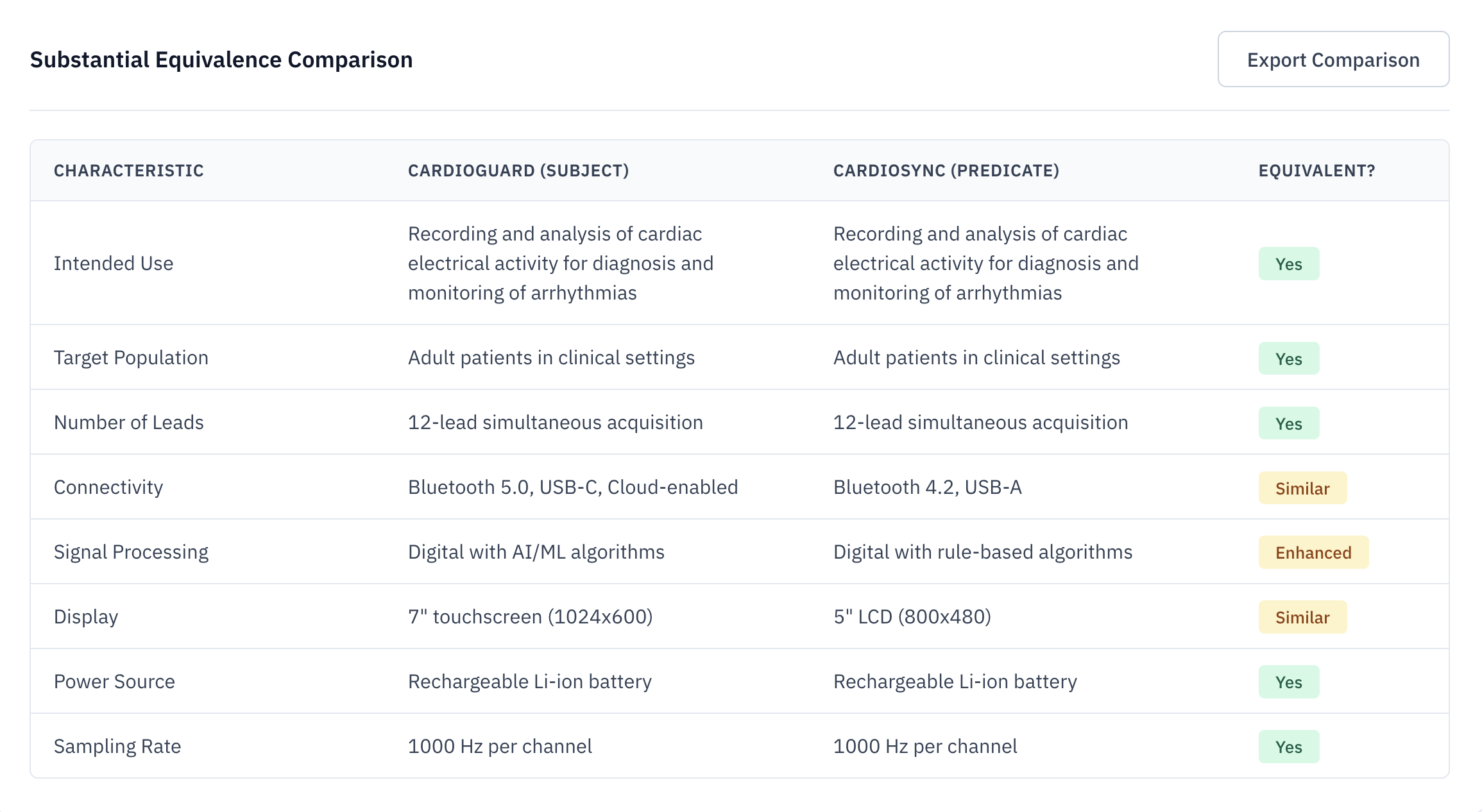
Substantial Equivalence
Automated side-by-side comparison of your device against selected predicates. Analyze indications for use, technological characteristics, and performance specifications.
- Side-by-side analysis
- Difference flagging
- FDA-ready matrix
Gap Analysis & Strategy
Identify regulatory gaps with specific testing recommendations (ISO 10993, IEC 60601) and generate strategy reports in eSTAR-compatible formats.
- Standards mapping
- eSTAR compatibility
- Automated reporting

Export & Integration
Generate submission-ready documentation in formats that work with your existing regulatory workflows.
PDF Export
PDFProfessional, formatted reports ready for regulatory review and internal archival.
- Audit trails included
- Formatted for review
- High-fidelity layout
Word Documents
DOCXFully editable documents for further customization and integration into your submission.
- Editable content
- Template-friendly
- Regulatory style guide
Structured Data
CSVClean data exports for deep analysis and integration with your existing R&D tools.
- Clean data mapping
- Tool agnostic
- Raw evidence data
All exports are designed to be eSTAR-compatible and include complete audit trails with FDA source citations, ensuring seamless integration into your final submission.